BLOG
learn more about Biotécnica
through our blog.
1. Contextualization
In December 2019, the world was surprised by a new infection with no defined etiologic agent located in Wuhan, China. This infection caused pneumonia that led to rapid depreciation of the respiratory function, progressing to acute respiratory syndrome (SARS). Severe Acute Respiratory Syndrome) (1). In January 2020, it was described that the etiologic agent of the reported pneumonia was from the family Coronaviridae, receiving the associated pathology the name of coronavirus disease (COVID-19) and the etiologic agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2, 3). In the months from January to March, the virus spread throughout the world, leading the World Health Organization (WHO) to declare on March 11, 2020, a pandemic state caused by SARS-CoV-2 (4). What contributed to the virus causing a pandemic is the fact that it is transmitted through the air, in addition to the fact that asymptomatic infected people can also transmit it (5). Once inside the organism, the virus promotes interaction with the plasma membrane of the human organism's cell through a surface glycoprotein called “spikes” that interacts with the angiotensin-converting enzyme receptor 2 (ECA2), so that it can enter with its genetic material inside the cell and start the viral replication process (6). The conclusive diagnosis of SARS-CoV-2 infection is performed using the real-time polymerase chain reaction (RT-PCR) technique Real Time - Polymerase Chain Reaction) (7), but because it is an expensive technique and requires scarce resources in countries like Brazil, several patients remain without the correct diagnosis. Thus, the objective of this work is to evaluate possible laboratory findings described in the literature that may corroborate with the patient's clinic, in order to facilitate the presumptive diagnosis of the infection caused by SARS-CoV-2.
2. Tests for the diagnosis of SARS-CoV-2 infection
2.1 - Molecular tests:
According to the WHO protocol, nucleic acid amplification tests (NAAT) Nucleic Acid Amplification Test), such as the RT-PCR technique, should be used to diagnose SARS-CoV-2 infection (8). Thus, the entity recommends that patients from areas with known circulation of the virus, as is the case in Brazil, are considered positive when they present a positive result for the amplification of only one target region of the viral RNA.
For RT-PCR testing, the Center for Disease Control (CDC) Centers for Disease Control and Prevention), recommends that samples should be collected using a swab in the oropharynx and nasopharynx regions (9). The CDC's recommendation is based on extensive literature, which clearly demonstrates that positive samples from the sites mentioned above have excellent assertiveness regarding infection caused by family viruses. Coronaviridae (10-12).
Looking for other samples that can be used in NAAT tests, the study by Xie et. al., compared the results obtained through stool, urine and blood samples with the results obtained by oropharyngeal swab. Although the study evaluated a positive population of only 9 patients, the study showed that only stool samples had a good correlation with the results obtained by oropharyngeal swab, with the same positivity being found in 8 of the 9 patients analyzed. Blood and urine samples did not correlate with the results of the oropharyngeal swab analyzes (13).
2.2 - Serological tests
The antibodies produced against the infection caused by SARS-CoV-2 begin to be observed after the symptoms, with immunoglobulin M (IgM) being detectable after the seventh day of infection and immunoglobulin G (IgG) after the fourteenth day of the onset of the infection. infection, as shown in figure 1.
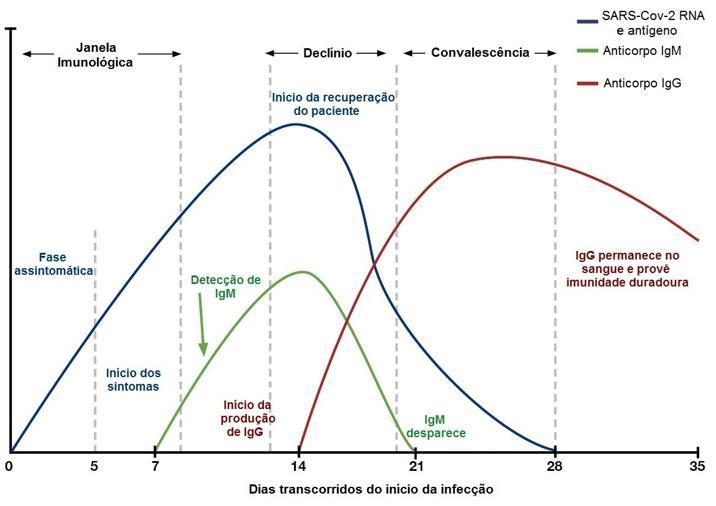
Adapted from Dyazime (14).
Given the scheme represented in the figure above, we can assess the progression of the disease in patients by associating the results of the antigen and antibody analysis. The diagnostic possibilities are shown in table 1.

2.3 - Conventional tests with predictive value for SARS-CoV-2 infection:
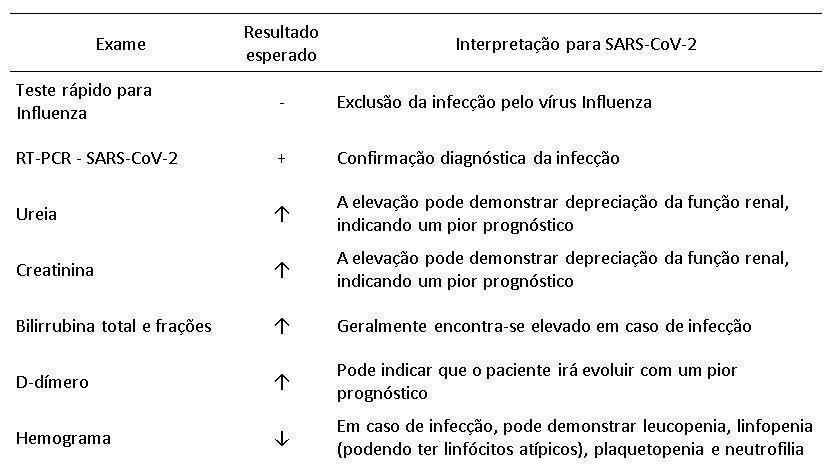
The Ministry of Health, through the Secretariat of Science, Technology, Innovation and Strategic Inputs in Health, published on April 6 of that year a document entitled “Guidelines for the diagnosis and treatment of COVID-19”, where it is possible to find a series of systematic reviews using the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) (15). Based on the articles selected through these reviews, the document suggests that patients with suspected SARS-CoV-2 infection can be evaluated with certain laboratory tests that, although they do not have specificity to diagnose the infection, assist in the differential diagnosis. The tests, as well as the interpretation of their respective results, are compiled in table 2.

3. Considerations about the diagnostic scenario:
Although molecular tests are considered the gold standard for the diagnosis of infection caused by SARS-CoV-2, the low availability of the tests, coupled with the centralization in some reference laboratories, leads to several pre-analytical complications that may imply in the quality of the test. result. An Italian study listed several possibilities of error that can happen during the process, which includes everything from the collection of material for molecular analysis, to the accuracy of the exam, highlighting that the stocking of the sample must be strictly controlled as it is one of the main sources of error of the process (16). WHO, through the guide entitled “Laboratory testing for coronavirus disease (COVID-19) in suspect human cases ”, presents instructions for the storage of several samples, especially for a sample collected by nasopharyngeal or oropharyngeal swab, which must be stored at -70OC when the sample will take more than 5 days to be analyzed (17).
Serology has a fundamental role in this context, especially because it is more accessible than molecular techniques, with rapid tests as the main form of presentation. However, several reports have been linked about failures in rapid tests, leading the Ministry of Health to prepare a document that compiles data such as sensitivity, specificity and precision extracted from the instructions for use of the various tests that were registered with ANVISA until the first half of April 2020 (18). It is possible to assess that there is variation between the performance of the tests, although, in general, the sensitivity of the serological tests is greater than 85% and the specificity, greater than 94%. As highlighted by the document, low percentages of sensitivity can lead to false-negative results, implying the lack of diagnosis of a patient truly affected by COVID-19.
As the offer of molecular biology and serology tests is scarce in several countries, including Brazil, there is a great effort in the search for other tests that can predict infection by SARS-CoV-2 (19, 20). As shown in Table 2, some changes in analytes have been described in the literature that can help when added to the radiological findings and the patient's clinic. Since these are non-specific tests, ruling out other causes such as infection by the Influenza virus is essential to increase the value of the test results.
The increase in urea and creatinine values may indicate a reduction in the glomerular filtration rate due to impaired renal function, which is a laboratory finding that indicates a greater possibility for the patient to evolve with a poor prognosis, and may even die, as demonstrated by Cheng et . al. (21). Associated with the change in urea and creatinine values, the increase in bilirubin levels, the change in coagulation reflected by longer periods of PT and aPTT, the inflammation demonstrated by the values of procalcitonin, protein C-reactin and milk dehydrogenase have a great predictive value of worsens in the patient's condition when evaluated together (22, 23).
D-dimer dosage, although not widely available in Brazil, is more accessible than the RT-PCR technique and is associated with more severe forms of SARC-CoV-2 infection, especially because some patients may develop thromboembolism pulmonary (24, 25). The increased D-dimer dosage is correlated with the decrease in PT and aPTT times, which indicates a worse prognosis for the patient (26). Like D-dimer, ultra sensitive Troponin I indicates a worse prognosis, mainly because it is associated with cardiac involvement by COVID-19 (27).
Changes in the blood count are seen with leukopenia, lymphopenia, thrombocytopenia and neutrophilia. These variations in cell count, combined with an increase in inflammatory cytokines such as interleukin-6, are also associated with a worse patient prognosis (28). The finding of lymphopenia is unusual because it is a viral infection, but the work of Tan et. al. proposes four hypotheses to explain lymphopenia, the first being that SARS-CoV-2 infects the lymphocyte and therefore decreases its quantity; the second that the virus attacks lymphoid organs such as the thymus and spleen; the third that cytokine disorder leads to lymphocyte apoptosis; the fourth that there is inhibition of lymphocyte metabolism (29).
Conclusion
Due to the lack of molecular and serological tests to confirm the infection of the new SARS-CoV-2, other laboratory tests may be of value when added together and with radiological and clinical findings presented by the patient. At a time when the resources then lacking, it is vitally important that the diagnosis is discussed among all professionals involved in patient care, so that the variables can be minimized and the presumptive diagnosis is the closest to reality.
References
- World Health Organization. Pneumonia of unknown cause - China 2020. Available from: https: //www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/.
- World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it 2020. Available from: https: //www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- World Health Organization. National capacities review tool for a novelcoronavirus 2020. Available from: https: //www.who.int/publications-detail/national-capacities-review-tool-for-a-novelcoronavirus.
- World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 2020, March 11. Available from: https: //www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Military Medical Research. 2020; 7 (1): 11.
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of advanced research. 2020; 24: 91-8.
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020; 25 (3).
- World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases 2020, march 19. Available from: https: //www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117.
- Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) 2020, April 8. Available from: https: //www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html.
- Poon LL, Chan KH, Wong OK, Yam WC, Yuen KY, Guan Y, et al. Early diagnosis of SARS coronavirus infection by real time RT-PCR. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2003; 28 (3): 233-8.
- Hui RK, Zeng F, Chan CM, Yuen KY, Peiris JS, Leung FC. Reverse transcriptase PCR diagnostic assay for the coronavirus associated with severe acute respiratory syndrome. Journal of clinical microbiology. 2004; 42 (5): 1994-9.
- Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clinical chemistry. 2020; 66 (4): 549-55.
- Xie C, Jiang L, Huang G, Pu H, Gong B, Lin H, et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2020; 93: 264-7.
- Diazyme Laboratories I. Why Do We Need Antibody Tests for COVID-19 and How to Interpret Test Results 2020. Available from: http://www.diazyme.com/covid-19-antibody-tests.
- Ministry of Health. Guidelines for the diagnosis and treatment of COVID-19 2020, April 6. Available from: https://portalarquivos.saude.gov.br/images/pdf/2020/April/07/ddt-covid-19.pdf .
- Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clinical chemistry and laboratory medicine. 2020.
- World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases 2020, March 2. Available from: https://apps.who.int/iris/handle/10665/331329.
- Secretariat of Science T, Innovation and Strategic Inputs in Health - SCTIE. Accuracy of diagnostic tests registered for COVID-19 2020, April. Available from: https://coronavirus.ceara.gov.br/wp-content/uploads/2020/04/NT_acuracia_diagnostico_COVID-19.pdf.pdf.
- Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive care medicine. 2020; 46 (4): 579-82.
- Guan WJ, Ni ZY, Hu Y, Liang WH, or CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020.
- Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney international. 2020.
- Napoli. MCMRACSCDRD. Features, Evaluation and Treatment Coronavirus (COVID-19). 2020, March 20.
- Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. Jama. 2019; 321 (20): 2003-17.
- Lippi G, Favaloro EJ. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thrombosis and haemostasis. 2020.
- Xie Y, Wang X, Yang P, Zhang S. COVID-19 Complicated by Acute Pulmonary Embolism. Radiology: Cardiothoracic Imaging. 2020; 2 (2): e200067.
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of thrombosis and haemostasis: JTH. 2020; 18 (4): 844-7.
- Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Progress in cardiovascular diseases. 2020.
- Cao X. COVID-19: immunopathology and its implications for therapy. Nature reviews Immunology. 2020.
- Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal transduction and targeted therapy. 2020; 5: 33.
HIGHLIGHTS


Privacy Overview
| Cookie | Duração | Descrição |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the "Analytics" category. |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the "Functional" category. |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies are used to store the user consent for the cookies in the "Necessary" category. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the "Performance" category. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
WhatsApp us
